During the COVID-19 pandemic, our healthcare industry faced tremendous challenges. The hard work of the front line workers, healthcare staff, and national administrations saved countless lives globally and earned the respect and gratitude of millions.
Fortunately, through the turmoil and after-effects of the pandemic, in under a year, vaccines were approved for administration to the public. However, this left healthcare industries with a major logistical challenge; organizing a vaccine distribution plan that would be safe and effective.
The vaccine distribution and shipment process must be well thought out and enacted in a timely manner. DHL estimated that it would need 15 million shipments, including 15 thousand flights, over the next 2 years to complete this task. IoT will be a crucial element to the safe distribution of vaccines through these flights and in all global distribution means.
The Centers for Disease Control and Prevention (CDC) in the U.S. states that certain vaccines must be stored at temperatures between -80°C and 60°C (-112°F to -76°F). If the vaccines are not stored at their specified temperature ranges, they will be considered a “temperature excursion” and may not be viable for use.
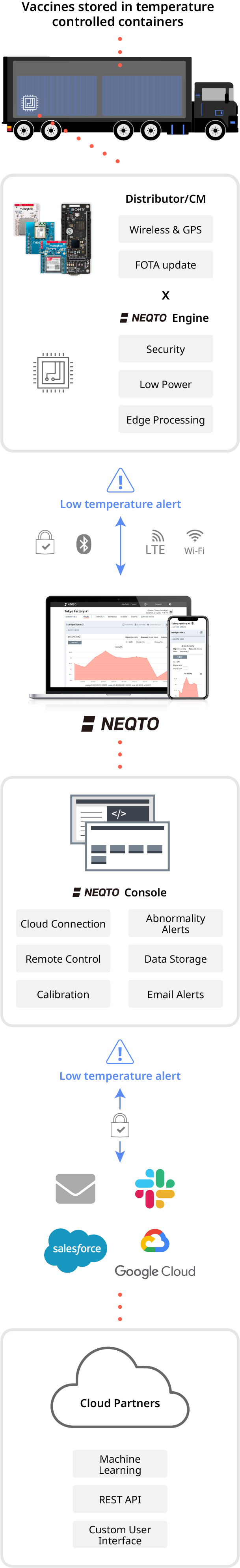
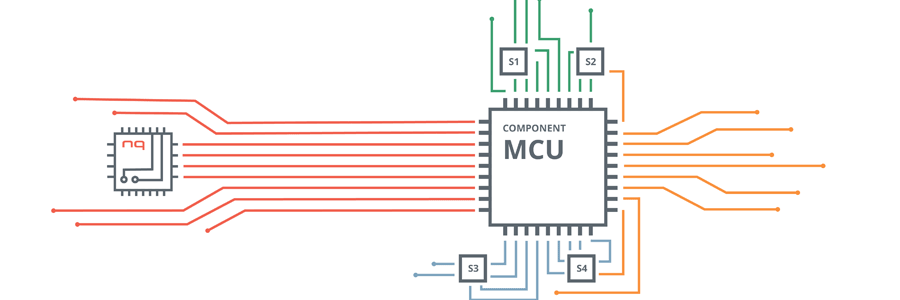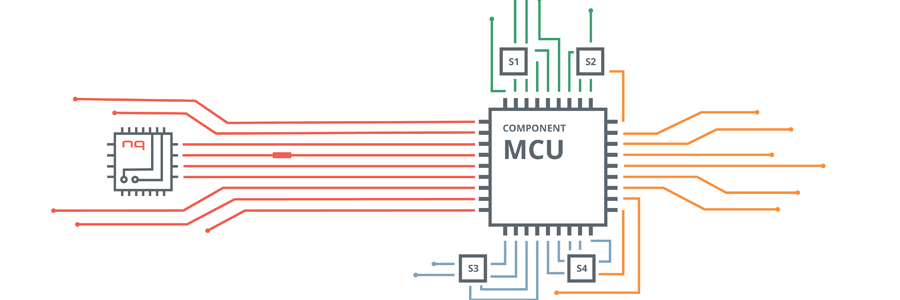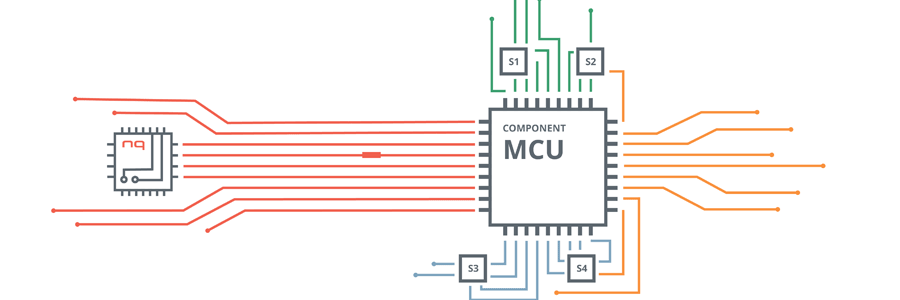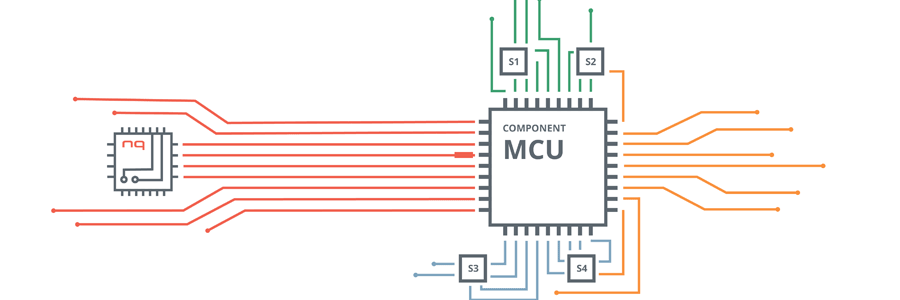
With the NEQTO solution, the vaccine manufacturers and the rest of the cold chain facilitators can control and trace the vaccine storage containers while they are transported to pharmacies, hospitals and clinics.
Facilitators are able to set the temperature range for the containers to trigger alerts and notifications if the temperature is outside of the temperature excursion range.
Consequently, vaccine manufacturers can then send a control message to the container to reduce or increase the vaccine storage temperature. Temperature regulation may also be triggered automatically via NEQTO’s low power edge processing functionalities and scripting capabilities.
In this study, the tracking and tracing of the entire cold chain operation is crucial to the safe preservation of the vaccines. Traceable records (i.e time-stamped temperature readings and other environmental, or sensor detected fluctuations or alerts) can be stored for future audits and used as proof of viability from the source to the final destination.
With these records, we will have the confidence to claim that all of the vaccines transported are viable and will be effective preventative treatments for the people receiving them.
NEQTO has partnered with a plethora of the best companies worldwide. With these partnerships, and the aforementioned points, we are prepared for quality assured COVID-19 vaccine distributions, and, in the future, for all other vaccines and medicines that require similarly precise temperatures to remain effective.